NEW CLICK HERE FOR THE AMAZON PRIME PAGES!
NEW CLICK HERE TO SEND US A GIFT! A COOL IDEA TO HELP US FOR HELPING YOU!
CLICK HERE FOR THE MAIN IOWA GOLD START PAGE
NEW IOWA GOLD FORUM WHERE TO GO, BUY SELL AND TRADE, EVENTS IN IOWA
NEW!! GOLD HOW TO PAGES HOW TO BUILD YOUR OWN GEAR!!
NEW BIG GOLD HOW TO PAGES FULL OF PLANS AND TIPS AND TRICKS OF HOW TO FIND GOLD
WHERE TO FIND GOLD IN THE USA A STATE BY STATE GUIDE
TECH SPECS ON GOLD WHAT IT IS ETC.
GOLD EQUIPMENT REVIEW GEAR WE HAVE TESTED AND WHAT WE THINK OF THE PRODUCT
DETECTOR PAGES GOLD AND COINS OR TRASH AND TREASURE TIPS AND TRICKS AND HOW TO PAGES
GOLD EQUIPMENT QUICK FIXES COOL TIPS HERE AND MORE ON HOW TO SET UP GEAR AND MORE TIPS AND TRICKS
IOWAGOLD NATURE AND TRAVEL PAGES!
THIS PAGE IS FOR INFORMATION ONLY
MERCURY IS NOT THE WAY TO RECOVER GOLD!!
HOW TO USE MERCURY
TO RECOVER GOLD
LEGAL NOTICE
the author shall not be liable for incidental or consequential damages in connection with or arising out of the furnishing or use of this material. I have no control over how you do these procedures. This procedure works for me and if something gets messed up it is your problem, not mine!
WARNING
- The processes contained herein require the use of high heat, mercury and very dangerous
acids, and must be performed in a well ventilated area. Always use mercury, sulfuric acid
and nitric acid in a well ventilated area. DO NOT breathe the fumes.
- Mercury begins to vaporize at room temperature and its fumes can be deadly.
- Fumes from many ores are deadly when heated.
- Nitric acid can be absorbed through the skin causing nitric acid poisoning. WEAR RUBBER
ACID GLOVES. Always add acid to water, NEVER ADD WATER TO ACID!
- Mercury and nitric acid can kill if swallowed.
- Nitric acid can ruin your clothes and shoes.
- Always wear rubber gloves, plastic safety glasses and a plastic or rubber apron.
IF YOU DO NOT UNDERSTAND ALL OF THE ABOVE WARNINGS, DO NOT GO ANY FURTHER!
EQUIPMENT USED
- Large copper gold pan
- Rubber spatula
- Tweezers
- Oral syringe or large veterinarian's syringe
- Virgin cotton balls
- Pyrex Beaker
INGREDIENTS USED
- Mercury
- Nitric acid
- Distilled water (With no chlorine)
- Mercury
NOTES
Any clean water without chlorine. Chlorine mixed with nitric acid can dissolve gold. Gold must be clean in order for mercury to attach itself. Sometimes placer gold will be covered with a thin film of oil, which will prevent the gold from being amalgamated unless the oil is cleaned off first. CAUTIONS: Working with nitric acid, can be very dangerous. Be extra careful to avoid spilling it on yourself or splashing it in your eyes. DO NOT breathe its fumes! When a solution of nitric acid is poured onto a dirty set of concentrates, the effect will be a bubbly reaction. Allow the concentrates to bathe until all such visible reaction has stopped.
PROCEDURE
1. Soak the concentrates in a 10:1 solution of nitric acid, which means 10
parts of water to 1 part of nitric acid. use a Pyrex beaker. This is to clean the gold.
You can do a better job of this if you put the concentrates and the 10:1 solution in a
rock tumbler with a plastic or rubber barrel (no metal, acid will corrode the metal).
2. Rinse the concentrates with fresh water so that the acid is diluted and washed away.
Once this is done, the concentrates are properly set up for amalgamation.
3. Take a clean, large, copper gold pan and coat thoroughly with mercury, using a pad of
folded cloth. Deposit the concentrates in the pan, add some fresh water and swirl and
agitate until all visible gold has been taken in by the mercury. If you want to check for
platinum, if you suspect it may be present, wash the black sands into a separate pan which
can be checked later.
4. Using a rubber spatula, scrape the gold bearing mercury from the copper gold pan into a
Pyrex beaker.
5. Wet a ball of virgin cotton and squeeze out the excess water. Place it into the bottom
of an oral or veterinarian syringe and pour in the amalgam ball. Replace the plunger and
holding the end of the syringe over a container, press the plunger to extract the excess
mercury. If the container is filled with water, the mercury will be prevented from
splashing or bouncing out as it drops into the container if you hold the end of the needle
under the surface of the water.
6. Remove the plunger from the syringe and extract the cotton containing the amalgam,
using tweezers. Put the amalgam ball into a Pyrex beaker and set it in a safe place,
downwind of any populated area within the vicinity.
7. Mix and pour in a solution of nitric acid and allow it to bubble until there is no
visible reaction. BE CAREFUL NOT TO BREATHE THE FUMES GIVEN OFF BY THE CHEMICAL REACTION!
8. Pour off the acid solution into another glass jar or a beaker; so that, the mercury in
solution can be recovered later (see how to do below).
9. If all of the mercury has not been dissolved from step 7, with the gold back in its
natural flake and powder form, pour fresh water into the jar and use an old screwdriver to
poke it around and break it up. Pour out the water and pour in another solution of nitric
acid. Sometimes it is necessary to poke at the gold just a bit to break it up while it's
being worked on by the acid. An old screwdriver works well for this.
10. When the reaction stops, flush with fresh water. If the gold is still not back in its
natural form, repeat the above steps. When dealing with small amounts of amalgam, usually
the gold will be thoroughly cleaned of it after step 7. Sometimes when working with larger
amounts of amalgam, it is necessary to do the steps a few times as described above, or to
use a stronger acid solution. NOTE: if you have a large amount of concentrates, you may
wish to ignore steps 3 and 4 and place the concentrates and an estimation of the correct
amount of mercury into a rock tumbler and allow it to turn for several hours. Some large
scale operations employ the use of portable cement mixers. If a new cement mixer is used,
run it first with a full load of sharp sand and gravel for 10 to 12 hours to scour out any
paint that may be present, as it will contaminate the mercury.
RECOVERING THE MERCURY
FROM THE NITRIC ACID SOLUTION
To recover the mercury in solution (see step 8), simply drop some aluminum foil into the acid solution. A chemical reaction takes place and the acid solution will drop the mercury to attack the aluminum. This causes the mercury to revert to its natural liquid metal form at the bottom of the jar. Then rinse out the acid solution and you will be left with most of your original mercury.
CLEANING MERCURY
After mercury has been used a number of times in the process of amalgamation, it becomes dirty and tends to break down into smaller, separate balls instead of it all coming together into a single mass. To clean dirty mercury, you simply soak it in a nitric acid solution of 30:1 part of acid. This will clean the impurities out and allow it to amalgamate properly again. Mercury can be used over and over to amalgamate and cleaned when necessary in this way.
ALSO TRY THIS TO REMOVE EXCESS MERC FROM AMALGUM;
All plastic syringe is a must for filtering mercury. Used for cleaning dirty mercury or removing gold from amalgam. Uses standard cotton balls as filters.
try a brass or plastic screen at the end of the plunger reciever to hold the cotton in place.
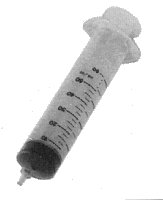
cool idea!!
in third world country's they just use a chamois and place the amalgum and beat it in a metal pan, twisting the chamois tight to press out the merc.
then they use a old cast iron skillet to drive off the merc....
the fumes are a real killer!! this method is not to be done!! it may cost you your life!!
there is the baked potato method also not to be done. core out a small section of the center of the tater place the amalgum in the center of the potato. and bake on an open fire. make sure you are up wind!! after cools the gold will be there and the potato is to be discarded.




![[Most Recent Quotes from www.kitco.com]](http://kitconet.com/images/quotes_7a.gif)